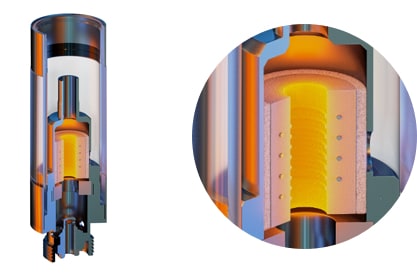
VapePod is disrupting the medical cannabis market with a quality, medically certified delivery system that works synergistically with patented, precision-targeted formulas. This comprehensive offering is designed to provide trusted relief to those suffering from a variety of disorders.
The VapePod vaporizer is produced in a certified GMP medical facility in full compliance with ISO13485, setting a new medical standard, engendering trust and ensuring peace of mind for our global clientele. The device was approved in 2018 as a medical device for cannabis extracts by the Israeli Ministry of Health, and is the first-ever medically certified vaporizer for the delivery of cannabis based formulas.