Kanabo has set up an R&D lab in the Weizmann Science Park. The company has obtained the necessary Cannabis R&D licenses, and has partnered with analytical labs to conduct third-party independent testing for all products. To date, Kanabo has developed a proprietary, patent-pending formula for sleep disorders (insomnia); three proprietary CBD formulations for Reload, Relax, and Repair and the VapePod vaporization delivery device. Kanabo has already conducted pre-clinical trials on the sleep disorders formula. Additional pre-clinical and medical validation activities are underway and in continued development at the R&D lab and through a CRO partner. This includes preparation for clinical trials at one of the leading hospitals in Israel. U.S. patent-pending for the sleep disorders formula through a pharmaceutical patent firm.
R&D Labs
Pre-Clinical tests
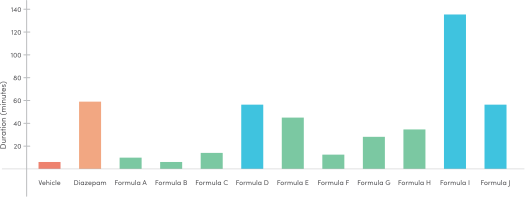
Successful Pre – Clinical Study on Sleep Disorder Formula
The current pre-clinical study dealt with vaporizable compositions comprising of compounds found in the cannabis plant (CBN, CBD, THC and certain terpenes). The purpose of the study was to test these compositions in treating sleep disorders.
The cannabinoids were tested separately for dose response, as well as different combinations to test for synergistic effects. Both sleep onset and sleep duration were documented, using the loss of righting reflex model in mice.
Several Test Items have been found to prolong the sleep duration compared to vehicle. In regard to sleep latency – a clear trend was not observed, and this aspect should be further investigated before any conclusions can be drawn. Based on the results of this study, these Test Items could be chosen for a larger scale continuous study.
Study Goals:
- Showing increase in efficacy, correlated with the predicted “Entourage Effect”
- Demonstrating increase in sleep maintenance and duration
The study was divided into different stages:
- Sleep duration was measured against the standard positive control (Diazepam 2mg/kg)
- Evaluation of a single cannabinoid’s effect, in a dose-response manner
- Evaluation of multiple cannabinoids in different doses
- Evaluation of multiple cannabinoids combined with terpenes
Safety & QA
In an effort to create a standard in the cannabis industry, our mission is to use only the most current technologies and work closely with manufacturers to develop the most innovative and effective delivery options. Kanabo’s VapePod and formulas have undergone a series of tests to ensure that we provide consumers with the safest and most effective device coupled with high-potency, broad spectrum, cannabis extracts.
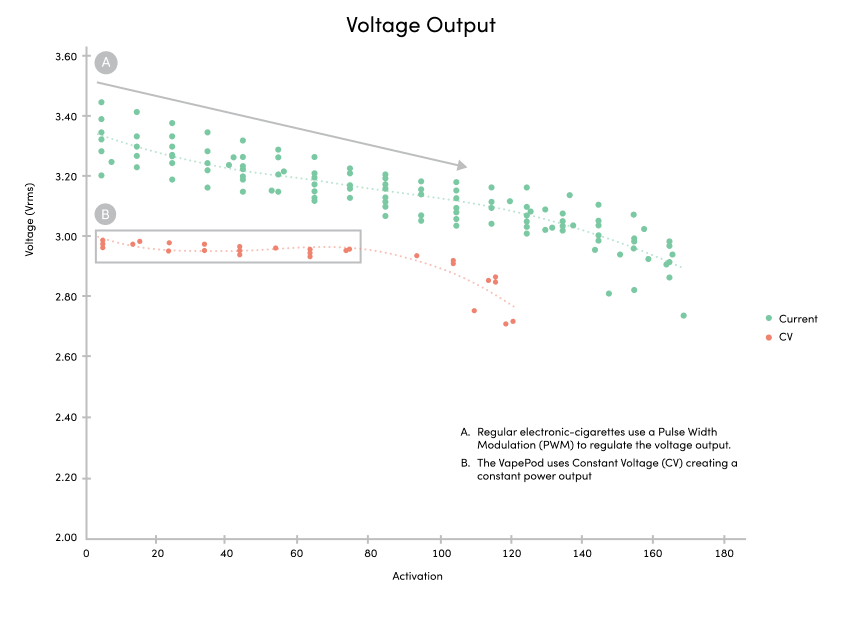
VapePod is powered by a universally rechargeable, breath-activated battery, with haptic feedback replacing traditional LED alerts for discreet operation. The basis of these tests were to determine the validity of the battery voltage, ensure accurate dosing, and to see that the formulas are not compromised in any way. The VapePod provides consistent and constant heat with little to no variation.
The result of our tests concluded that the VapePod’s battery voltage provided steady heat deriving a consistent dose.
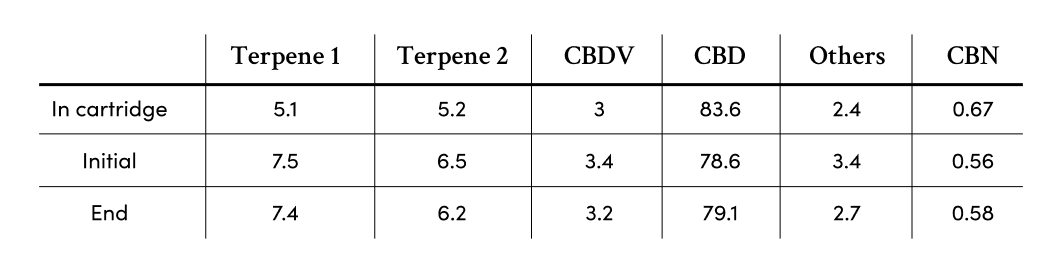
Dosing
The ability to replicate the same dose engenders trust in our device. The VapePod is able to offer a dose with consistent accuracy of 1.2 mg
The results of our tests concluded that the battery was able to replicate each inhalation with the same amount of vapor.
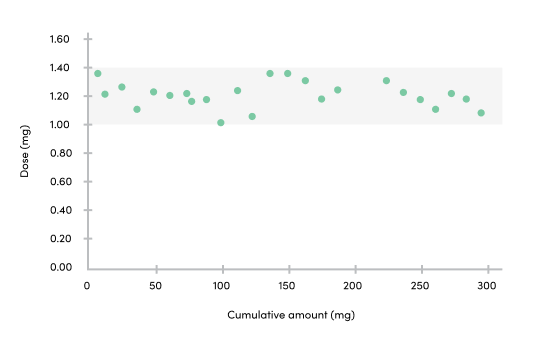
Consistency
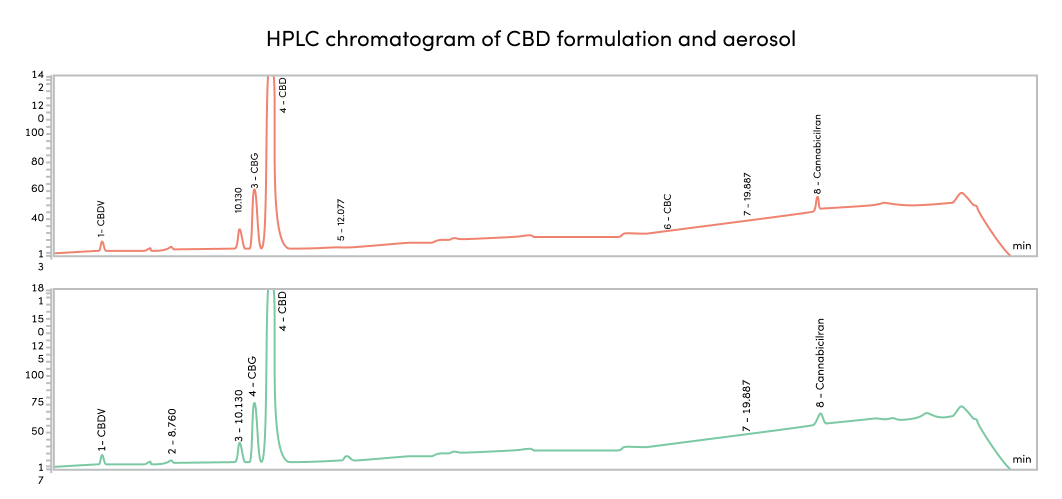
We provide our consumers with a specially designed formula that has no variation when heated. Further building Kanabo’s standard of quality, the vapor is then tested for consistency as heating has the potential to unlock unwanted byproducts.
The result of our tests concluded that the quality of the vapor produced by the VapePod remained consistent with little to an insignificant amount of byproducts. No matter how full or depleted the cartridge was, the vapor remained consistent both in cannabinoids and terpenoid composition.